—How practical methods and interpretations of composition evolved in eighteenth-century chemical analysis.—
Composition is a central aspect of modern chemistry. Various substances are identified by some aspect of their elementary composition, whether it’s by the proportions and identities of the elements in a salt’s chemical formula or the way in which those elements are spatially arranged in an organic substances’ molecular structure. However, the view according to which chemical species can be characterized and classified according to composition did not always exist, and rather emerged within chemists’ manipulation of different substances in their laboratories.
Traditional historical accounts, including a famous paper by Robert Siegfried and Betty Jo Dobbs, have associated the emergence of this view of chemical composition with the chemical revolution of the late eighteenth century. During this time, Antoine de Lavoisier (1743-1794) and his French colleagues developed a new system of chemistry in which substances were named and classified on the basis of their composition, proceeding from the simplest to the more complex. The system was centered on a list of chemical elements or “simple substances”, indecomposable bodies that definitively replaced the four elements as the constituents of all other chemical substances. One of the novel aspects, according to Siegfried and Dobbs, was that Lavoisier explicitly based his conclusions regarding composition on the experimental methods of analysis and synthesis, for example through the public demonstration of his ability to decompose and reproduce water in 1785. He characterized chemical elements as substances that should be obtainable through chemical operations, as “the last point which analysis is capable of reaching”. However, ever since the 1970s, historians have questioned the revolutionary nature of Lavoisier’s work by showing how an ‘analytical’ view of chemical composition gradually developed over the course of the eighteenth century and continued to evolve into the nineteenth. In this text, I will present some of the aspects of that gradual development and explain how they were connected to the evolution of analytical methods.
Over the course of the eighteenth century, a relatively standardized methodology of chemical analysis emerged at the intersection of academic, artisanal and industrial knowledge. Following a preliminary description that included the colour, shape, texture and taste of a substance, this method included so-called ‘wet’ and ‘dry’ methods aimed at determining both the nature and the proportion of the components. The former referred to the study of mineral substances in solution, whereas the latter described the procedures of assaying carried out at a high temperature (such as fusion, calcination or vitrification). Many of these operations originated in sixteenth- and seventeenth-century Europe as part of the main chemical arts of that period, pharmacy and metallurgy, and they enabled chemical practitioners to identify and decompose a large number of salts, alloys and aqueous solutions.

Engraving of a laboratory and an affinity table copied from the chemistry course of Rouelle. Encyclopédie, planche 1ère, Recueil des planches t. III «Chimie». ENCCRE, Édition Numérique Collaborative et CRitique de l’Encyclopédie.
The distinction between ‘wet’ and ‘dry’ methods emerged within the early eighteenth-century affinity tables, in which substances were classified and ordered according to their tendency to replace one another in a reaction. The practice of establishing affinity tables spread throughout Europe following the publication of the “Table des différents rapports observés en chimie entre différentes substances” by Etienne François Geoffroy (1672-1731) in 1718. It consisted in a systematic experimental study of composition focused on the ways in which substances could be separated and recombined which emerged within the context of salt chemistry. This approach is characteristic of eighteenth-century European chemistry, which was practiced at a variety of academic as well as non-academic sites and which mainly focused on studying commodities found in mines, workshops, distilleries or apothecary shops.
Over the course of the eighteenth century, analytical methods further developed, largely thanks to economic, technical and industrial activities. Various European governments stimulated their national industries and hired officials to research the composition and production of various materials. Likewise, many methods had their origins in artisanal and industrial practices: for example, Swedish and German-speaking chemical mineralogists of the mid-eighteenth century adopted the blowpipe, which had commonly been used by glassblowers and goldsmiths to melt glass and metals. One could use such a pipe to blow air onto a flame in order to change its direction and increase its temperature, thereby allowing for a portable, quick and cheap method to perform a simplified version of dry analysis that could be used to produce easily replicable results in the field.
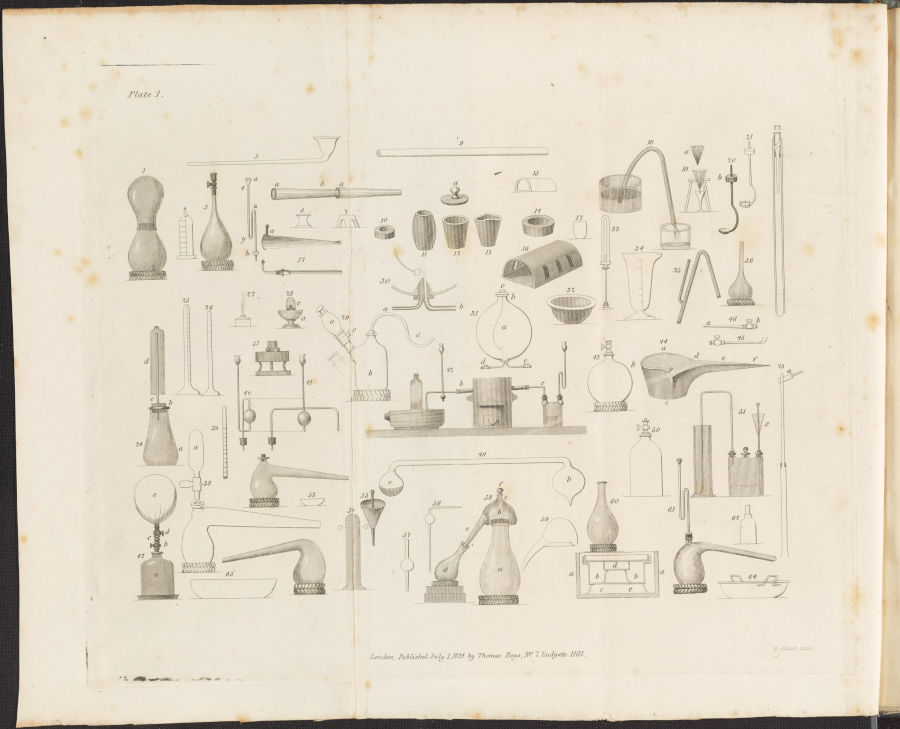
Engraving from Friedrich Accum’s Explanatory Dictionary of the Apparatus and Instruments Employed in the Various Operations of Philosophical and Experimental Chemistry (1824) which depicts various chemical instruments and apparatus, including a blowpipe (figure 17). Science History Institute Museum & Library, Digital Collections.
Similarly, wet methods were improved by the addition of new indicators, reagents and other tests by which to detect and identify the constituents of a mineral solution. These methods also improved thanks to chemical mineralogy, for example under the influence of Torbern Bergman (1735-1784) who systematized existing procedures and added new ones, but also thanks to many pharmacists who continued to study mineral waters. In France, for instance, Bergman’s methods enjoyed a high popularity as mineral waters increasingly came to be characterized on the basis of their chemical composition rather than medical effects. Eighteenth-century pharmacists also relied mostly on wet methods for the study of plant and animal substances, as they had come to rejected the use of fire as destructive of these substances’ components and rather preferred wet methods such as extraction which produced the unaltered constituents of plant and animal substances.

Lavoisier’s table of simple substances from his 1789 textbook. Gallica, Bibliotèque nationale de France.
In the context of these systematic studies of the ways in which chemical substances could be decomposed and recombined, chemical practitioners gradually adopted a new characterization of substances on the basis of their composition. While most historians agree that such a shift occurred, there has been much debate about when exactly the new view appeared. Lavoisier’s work, rather than revolutionary in itself, might therefore be viewed as an important step in the gradual rise of what Hasok Chang has called “compositionism”: a system of chemical practice which focused on experimentally studying composition and characterizing substances as either simple or composed of other substances.
For instance, David Oldroyd, Hjalmar Fors and others have argued that Swedish and German-speaking chemical mineralogists implicitly adhered to an analytical conception of chemical elements. In opposition to the Linnaean system which classified minerals on the basis of their shape and color, they adopted a classification on the basis of chemical composition following the systems of Bergman, Axel Fredrik Cronstedt (1745-1765) and Abraham Gottlob Werner (1749-1817), which was directly informed by chemical analysis. Rather than reflecting on ultimate composition, these practitioners adopted a pragmatic approach that focused on the constituents they were actually able to manipulate in the lab and which appeared as a kind of ‘chemical elements’ distinct from the four true elements.
Other historians of chemistry have pointed to even earlier occurrences of the view that characterized substances as either simple or complex. According to Ursula Klein, Geoffroy’s 1718 table implicitly contained the first instance of a new concept of chemical compound. The novelty of this concept, she argues, lay in the idea that stable, apparently simple, components could be combined to form chemical compounds and reproduced through decomposition of those compounds. This view differed from that of earlier practitioners who saw all composed substances as perfectly homogeneous and therefore did not clearly distinguish between simple and compound bodies. However, Klein’s view has also been criticized, mainly by William Newman who has instead argued that the ideas of reversible (re)combinations of stable components were already present in medieval and early modern alchemy. The works of Geoffroy’s teacher Wilhelm Homberg (1652-1715), studied by Mi Gyung Kim and Lawrence Principe, indeed directly connect the eighteenth-century concept of chemical compound to early modern chymical traditions. It might therefore be more appropriate to view this development as a gradual transition.
In the case of plant and animal substances, the rise of the compositionist approach does not appear as clearly. On the one hand, chemists did increasingly focus on determining the elementary composition of these substances, especially as the discipline of chemistry established itself as separate from pharmacy, medicine and natural history. On the other hand, however, elementary analysis had the disadvantage of breaking down these substances without the possibility of reproducing them. This difficulty motivated many pharmacists to rely on extraction methods in order to extract the immediate principles of plant and animal substances and conserve their medical properties. Although various methods for elementary analysis had been developed, such as Lavoisier’s combustion method, it remained difficult to determine the exact composition of plant and animal substances well into the nineteenth century. Sacha Tomic has argued that organic chemistry eventually emerged out of the convergence of immediate and elementary analysis: the difficulty of identifying the elementary composition of immediate principles such as alkaloids pushed the development of new analytical procedures and instruments, such as the Kaliapparat of Justus von Liebig (1803-1873), which are associated with the birth of organic chemistry in the 1830s. Recently, Catherine Jackson has shown how the subsequent development of this new discipline relied on the combined practice of organic analysis and synthesis, leading to the stabilization of theoretical views on molecular structure over the course of the nineteenth century.
Thus, the historical literature clearly shows how the evolution of chemists’ interpretation of chemical composition is completely intertwined with the development of their practical methods of chemical analysis. As their practices of decomposition, identification and recombination of substances stabilized, so did their theoretical conceptions about the ways in which substances were composed. Despite the various changes in chemical theory that took place at the end of the eighteenth century, many continuities between the eighteenth and nineteenth century can be identified.
One of them is the relative stability of the methods used for mineral analysis: although the practice lost in relative importance with the rise of organic chemistry from the 1830s onwards, the methodology of ‘classical analysis’ that emerged over the course of the eighteenth century remained the standard way to characterize mineral substances until the middle of the twentieth century. As Armel Cornu explains elsewhere in Sabers en Accio, these methods often relied on the use of the senses. Even as quantitative approaches gained in importance, chemists continued to rely on taste, smell, sight and texture for the identification of chemical substances.

Closeup image of glass jars in Grand Duke Peter Leopold’s eighteenth-century chemistry cabinet from the Museo Galileo in Florence, Italy. Museo Galileo.
Similarly, chemical classifications of the nineteenth century clearly bore the traces of eighteenth-century practices. This not only concerns specific classes such as acids and alkalis, which were inherited from salt chemistry, but also the more general practice of classifying substances by the patterns in their tendencies to combine with other substances. The use of such patterns, which were also called ‘chemical analogies’, for the identification of families of chemical substances emerged within the affinity tables and lasts until today in the columns of the periodic table.
Based on the idea that such similarities in properties were an indication of similarities in composition, chemical analogies were regularly used to predict the composition of indecomposable bodies during the nineteenth century. In other words, despite the explicit affirmation of Lavoisier and his followers that composition should be identified solely on the basis of direct decomposition attempts, more indirect indications were taken into account as well. The ‘analytical’ conception of chemical composition was therefore more centrally concerned with characterizing substances on the basis of their elementary composition, rather than progressively decomposing each body and isolating elementary substances. We might therefore conclude that chemical analysis was primarily aimed at the identification of composition, a question that required the integration of experimental and reasoning practices.
Sarah Hijmans
Université Paris Cité
How to cite this paper:
Hijmans, Sarah. Chemical analysis and composition. Sabers en acció, 2024-11-13. https://sabersenaccio.iec.cat/en/chemical-analysis-and-composition/.
Find out more
You can find further information with the bibliography and available resources.
Recommended reading
Chang, Hasok. 2011. «Compositionism as a Dominant Way of Knowing in Modern Chemistry». History of Science 49 (164): 247-68.
Holmes, Frederic Lawrence. 1989. Eighteenth-Century Chemistry as an Investigative Enterprise. Berkeley (Calif.): University of California at Berkeley.
Siegfried, Robert, and Betty Jo Dobbs. 1968. “Composition, a Neglected Aspect of the Chemical Revolution.” Annals of Science 24 (4): 275–93.
Szabadváry, Ferenc. 1966. History of Analytical Chemistry. Translated by Gyula Svehla. Oxford: Pergamon Press.
Studies
Abney Salomon, Charlotte A. 2019. “The Pocket Laboratory: The Blowpipe in Eighteenth-Century Swedish Chemistry.” Ambix 66 (1): 1–22.
Fors, Hjalmar. 2014. “Elements in the Melting Pot: Merging Chemistry, Assaying, and Natural History, Ca. 1730–60.” Osiris 29 (1): 230–44.
Hijmans, Sarah. 2023. “From Lavoisier to Mendeleev: The Identification of Chemical Elements in Practice Between 1770 and 1870”. PhD, Paris: Université Paris Cité.
Jackson, Catherine. 2023. Molecular World: Making Modern Chemistry. MIT Press.
Kim, Mi Gyung. 2000. Chemical Analysis and the Domains of Reality: Wilhelm Homberg’s Essais De Chimie, 1702–1709. Studies in History and Philosophy of Science 31(1): 37–69.
Klein, Ursula. 1994. “Origin of the Concept Chemical Compound.” Science in Context 7(2): 163–204.
Oldroyd, David R. 1975. “Mineralogy and the ‘Chemical Revolution’.” Centaurus 19 (1): 54–7.
Simon, Jonathan. 2002. “Analysis and the Hierarchy of Nature in Eighteenth-Century Chemistry.” British Journal for the History of Science 35 (1): 1–16.
Tomic, Sacha. 2010. Aux origines de la chimie organique: méthodes et pratiques des pharmaciens et des chimistes (1785-1835). Rennes: Presses universitaires de Rennes.
Sources
Geoffroy, Etienne François. 1718. “Table des differents rapports observés en Chimie entre differentes substances.” Mémoires de l’Académie royale des sciences, année 1718, Paris, Impr. Roy., 1741, p. 202-212. Translated into Spanish with notes in Klein Ursula, Grapí, Pere, García Belmar, Antonio. 2012. La representación de lo invisible: Tabla de los diferentes “rapports” observados en la química entre diferentes sustancias de E. Geoffroy. San Vicente del Raspeig: PUA. 122 p.
Lavoisier, Antoine. Traité élémentaire de chimie, présenté dans un ordre nouveau et d’après les découvertes modernes par M. Lavoisier. A Paris: chez Cuchet, 1789. https://gallica.bnf.fr/ark:/12148/bpt6k5524956g.
Rose, Heinrich. 1829. Handbuch der analytischen Chemie. 1st ed. Berlin: E. S. Mittler. https://books.google.com.ag/books?id=2f-9qkWHM0YC&printsec=frontcover&hl=es&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false.
Websites and other sources
BBC Radio In our time, Episode “Oxygen”, 15 November 2007. Accessed 20 November 2024. https://www.bbc.co.uk/programmes/b0088nql.
Cornu, Armel. “How do we know when water is safe to drink? The early modern origins of water analysis.” Accessed 5 July 2024. https://www.jargonium.com/post/how-do-we-know-when-water-is-safe-to-drink-the-early-modern-origins-of-water-analysis.
Hijmans, Sarah. “How to identify simple substances. Chlorine vs Oxymuriatic acid.” Accessed 20 November 2024. https://www.jargonium.com/post/how-to-identify-simple-substances-chlorine-vs-oxymuriatic-acid.
Lafont, Olivier. “Étienne-François Geoffroy et la Table des Affinités”, l’Actualité Chimique N°444-445, 2019. https://new.societechimiquedefrance.fr/numero/etienne-francois-geoffroy-et-la-table-des-affinites-p99-n444-445/?lang=en. Accessed 20 November 2024.



