—How and Why Mutagenicity Testing Became a Part of Environmental Science and Regulation since the 1970s.—
The Ames test was developed in the early 1970s by Bruce N. Ames (1928–2024) to detect whether chemicals cause genetic mutations in bacteria. It makes use of several mutant strains of Salmonella typhimurium that are deficient in synthesizing the essential amino acid histidine. These strains are normally unable to grow on minimal media, but the presence of a mutagenic chemical will induce mutations that restore a bacterial cell’s ability to make its own histidine. The original test examined growth of the Salmonella strains on a petri dish; each colony of cells represented a mutation. Next figure depicts the Ames test.
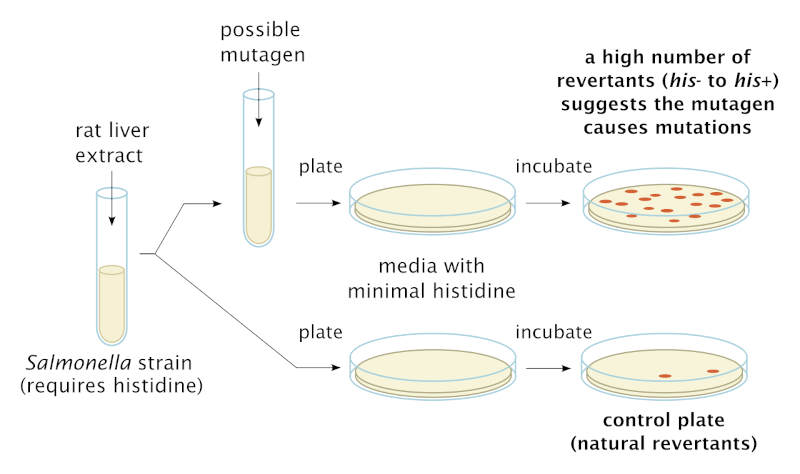
Schematic diagram showing the procedure of the Ames test using customized histidine-requiring mutant strains of Salmonella typhimurium. Wikimedia.
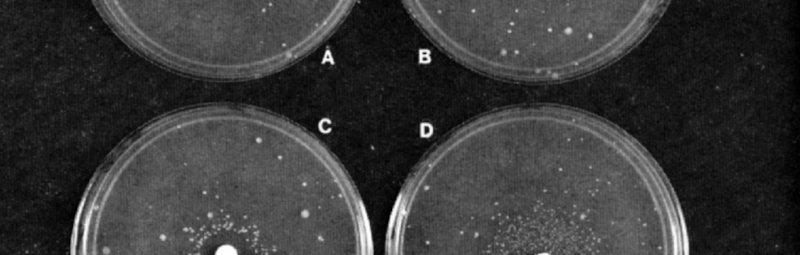
There is a natural background rate of mutation, hence a positive Ames test result depends on the appearance of more colonies than the control plate, without the substance being tested. The following figure shows Ames test results in which the test substance is added via a paper disk on the Petri dish agar. Ames added to the assay an extract of rat liver with enzymes known to activate many human carcinogens (cancer-causing substances). This means that the Salmonella cells are exposed to metabolic intermediates of the test chemical as they would be generated in the mammalian body. In general, a positive result on the Ames test is taken as indication that the chemical substance also causes mutation in animals, including humans.

Pictures of Ames Tests for (A) Spontaneous Revertants and exposure to (B) Furylfuramide, (C) Alfatoxin B1, and (D) 2-Aminofluorene. The mutagenic compounds in B, C, and D were applied to the 6 mm filter disk in the center of each plate. Each Petri plate contains cells of the tester strain in a thin overlay of top agar. (The strain used here is TA98, derived by adding a resistance transfer factor to a Salmonella tester strain, mutant hisD3052, that scores frameshift mutations.) Plates C and D contain, additionally, a liver microsomal activation system isolated from rats. The spontaneous or compound-induced revertants, each of which reflects a mutational event, appear in a ring as spots around the paper disk. Source: Ames, McCann & Yamasaki 1975, p. 358. ResearchGate.
The Ames test was one among dozens of short-term laboratory tests developed to identify agents that damage DNA. In fact, the Ames test only identifies single DNA base-pair changes or frameshift mutations. (The test uses several strains because each one registers a different mutational change to DNA at the level of individual bases, i.e., adenine, thymine, guanine, or cytosine.) Other tests for mutagenicity (i.e., the ability to induce mutations) were developed to identify genetic alterations on a larger scale, such as insertions, deletions, or chromosomal aberrations. The term genotoxicity refers to all of these mutational changes and may include epigenetic alterations. See next figure.
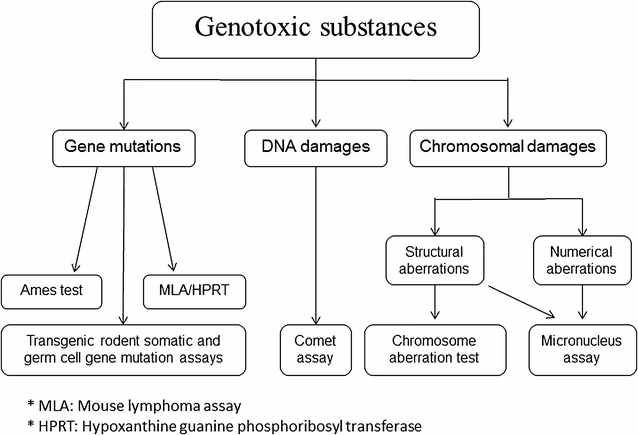
A schematic diagram representing the most common testing methods used to detect genotoxic substances. The Ames test is under gene mutations on the far left. Journal of Translational Medicine.
In the 1970s, US government agencies such as the Food and Drug Administration and the Environmental Protection Agency began developing guidelines for mutagenicity testing of new products under regulatory review. Modifications have been made to the Ames test since it was first introduced (such as growth of cells in culture tubes or automated colony counting), and a similar genetic reversion test developed in the bacterium Escherichia coli is sometimes also referred to as an “Ames test,” because it works by the same principle. Even as these simple tests using bacteria have been supplemented by genotoxicity tests using animal cells, the Ames test remains the most widely-used method for determining mutagenicity. To this day, Ames test data is ordinarily submitted to regulatory agencies that require toxicity data for authorization of new chemical products.
The introduction of the Ames test to identify mutation-causing chemicals responded to growing public concerns in the US about toxic substances in food, drugs, consumer products, and the environment. Through the twentieth-century, laboratory research and observations of worker sickness had suggested that industrial chemicals were linked to human cancer incidence, which appeared to be on the rise.
The 1958 “Delaney Amendment” of the US Food and Drug Administration (FDA) banned the use of carcinogenic food additives. Silent Spring, published by Rachel Carson in 1962, alarmed Americans with vivid depictions of how synthetic chemicals were destroying wildlife and threatening human health. Moreover, in the late 1950s and early 1960s, growing public opposition to atomic weapons testing because of the dangers of radioactive fallout prompted the US, USSR, and UK to sign a treaty banning further atmospheric tests. In the US, continued public concern about cancer from environmental radioactivity led to large-scale opposition to nuclear power plants. The environmentalist movement of the 1970s, catalyzed by Carson’s book, built on the public opposition to atomic weapons testing while focusing new attention on pesticides and pollutants.
Responding to these public concerns and the broader environmentalist movement, the US federal government targeted cancer from chemicals as a key public health problem. Whereas toxicity had traditionally been understood in terms of immediate (or acute) effects on an organism, scientists were increasingly worried about so-called chronic toxicity, or the long-term effects of chemical exposure, even at low doses. In 1968, the US National Cancer Institute (NCI) launched a “Plan for Chemical Carcinogenesis and the Prevention of Cancers.” During the subsequent years this agency publicized an estimate that as much as 90% of human cancers were due to environmental carcinogens. Carcinogens induced cancer, current thinking went, by inducing DNA mutations in somatic cells. This theory of cancer causation (one among many) dated to the early twentieth century, but during the 1950s and 1960s, expanding knowledge about the health hazards of ionizing radiation provided new evidence for it. Radiobiologists (often funded by the US Atomic Energy Commission) showed that no level of ionizing radiation was completely harmless; even low-dose exposures could induce cancer-producing mutations. In addition, the “multi-stage model” of carcinogenesis proposed by Peter Armitage and Richard Doll in 1954 treated cancer as the result of not just one, but several, cellular events. These events could be mutations, such as those induced by radiation—or by chemical pollutants.
If cancer could be viewed as a disease of mutations, then chemical carcinogens must be agents that induce mutations (mutagens). It was this line of thought inspired Berkeley biochemist Bruce Ames to develop his simple laboratory assay using customized strains of the bacteria Salmonella typhimirium. By the mid-1970s, his laboratory demonstrated that that in a sample of 300 chemicals, 90 percent of Salmonella mutagens were also rodent carcinogens, and that 89 percent of animal test carcinogens were also bacterial mutagens. In 1975, Bruce Ames and co-workers identified many hair dyes as mutagenic, a finding that was widely reported in the media. In addition, Arlene Blum and Bruce Ames identified as mutagenic a chemical flame retardant that had recently been added to children’s pajamas to meet anti-flammability standards. The substance (called Tris) was subsequently found to cause cancer in rodents. When the US Consumer Product Safety Commission banned this substance from children’s apparel, it cited mutagenicity results from the Ames test.

Photograph of Bruce N. Ames from 2009. Wikimedia.
As the example of Tris indicates, Ames’s rapid and inexpensive test became a major tool in toxicology, regulation, and environmental science. Pharmaceutical companies adopted it to identify potentially carcinogenic drugs. Since 1962, the FDA had required strict pre-market testing for all new drugs, and being able to identify possible carcinogens before conducting expensive animal tests was advantageous to industry. In addition, the US Environmental Protection Agency (EPA) and FDA began requiring Ames test data (or similar short-term mutagenicity testing) for new pesticides and food additives. Scientists and government officials expected that widespread use of the Ames test would enable comprehensive screening and regulation of all chemicals (there were already 60,000 on the market in the mid-1970s) to decrease or even eliminate the incidence of cancer from exposure.
This hope was not fulfilled. More chemicals tested positive as mutagens than initially expected, including many natural substances. In addition, lobbying by industry prevented inclusion of a requirement for mutagenicity testing for new chemicals in the US Toxic Substances Control Act of 1976. Although this statute is replete with the term “testing,” by the early 1980s most chemical companies did not report on mutagenicity or any other health and toxicological test data in premanufacturing notices for new products. In addition, federal agencies with oversight over chemicals (such as the EPA) are charged with protecting confidential business information (CBI). Companies generally identified toxicity data as CBI, preventing it from being publicly shared. In 1997, the Environmental Defense Fund reported that for the top-selling 3,000 chemicals in the United States, basic toxicity testing was unavailable to the public for more than 70 percent.
In the 1980s, the understanding of cancer as triggered by mutations, which had been so central to environmentalist health policies, was assimilated into an increasingly genetic framework. The Human Genome Project intensified the search for mutations associated with higher cancer risk, such as in the BRCA genes for breast cancer. Reflecting these trends, cancer prevention highlighted individual susceptibility and lifestyle, often at the expense of attention to involuntary environmental exposures. Physicians and public health officials increasingly spoke of cancer in terms of risk factors. Even environmental health scientists embraced this turn, with the emergence of “toxicogenomics” to study genetic variation among individuals in susceptibility to exposure.
Opponents of industrial regulation have mobilized these newer biological models of carcinogenesis, especially those related to genetic predisposition, to divert attention from the possible culpability of pollutants and commercial chemicals in human cancer. At the same time, the grounds for understanding toxic effects of industrial chemicals have shifted with the rising interest in endocrine disruptors, which may contribute to cancer but not via mutation.
Following the introduction and use of the Ames test reveals how scientists and regulatory authorities attempted to use science to protection human health and the environment by limiting control exposures to toxic chemicals. The history also shows how the chemical industry fought new regulatory requirements to this end, often through what Robert Proctor has called “trade association science.” In this respect, the debates over preventing cancer through controlling mutagenic chemicals were part of a broader struggle over the environmental costs of industrialization, including climate change due to the continued reliance on fossil fuels. Improving environmental protection depends on determining what counts as scientific knowledge, who has access to it, and how it informs regulatory decision-making. Standardized tests are critical yet often overlooked elements of environmental science and regulation.
Angela N. H. Creager
Princeton University
How to cite this paper:
Creager, Angela N. H. The Ames Test. Sabers en acció, 2025-07-09. https://sabersenaccio.iec.cat/en/the-ames-test/.
Find out more
You can find further information with the bibliography and available resources.
Recommended reading
Boudia, Soraya, Angela N. H. Creager, Scott Frickel, Nathalie Jas, Carsten Reinhardt, and Jody A. Roberts. 2022. Residues: Thinking Through Chemical Environments. Rutgers University Press.
Oreskes, Naomi and Erik M. Conway. 2010. Merchants of Doubt: How a Handful of Scientists Obscured the Truth on Issues from Tobacco Smoke to Global Warming. Bloomsbury.
Proctor, Robert. 1995. Cancer Wars: How Politics Shapes What We Know & Don’t Know about Cancer. Basic Books.
Studies
Boudia, Soraya, and Jas, Nathalie. 2013. Toxicants, Health and Regulation Since 1945. London: Pickering & Chatto.
Brickman, Ronald, Sheila Jasanoff, and Thomas Ilgen. 1985. Controlling Chemicals: The Politics of Regulation in Europe and the United States. Cornell University Press.
Creager, Angela N. H. 2014. “The Political Life of Mutagens: A History of the Ames Test,” in Powerless Science? Science and Politics in a Toxic World, edited by Soraya Boudia and Nathalie Jas, 46–64. Berghahn Books.
Creager, Angela N. H. 2021. “To Test or Not to Test: Tools, Rules, and Corporate Data in U.S. Chemicals Regulation,” Science, Technology & Human Values 46, no. 5: 975–97 https://doi.org/10.1177/01622439211013373
Demortain, David. 2020. The Science of Bureaucracy: Risk Decision Making and the US Environmental Protection Agency. MIT Press.
Frickel, Scott. 2004. Chemical Consequences: Environmental Mutagens, Scientist Activism, and the Rise of Genetic Toxicology. Rutgers University Press.
Langston, Nancy. 2010. Toxic Bodies: Hormone Disruptors and the Legacy of DES. Yale University Press.
Markowitz, Gerald, and Rosner, David. 2002. Deceit and Denial: The Deadly Politics of Industrial Pollution. University of California Press.
Murphy, Michelle. 2006. Sick Building Syndrome and the Problem of Uncertainty: Environmental Politics, Technoscience, and Women Workers. Duke University Press.
Sellers, Christopher. 1997. Hazards of the Job: From Industrial Disease to Environmental Health Science. University of North Carolina Press.
Shostak, Sara. 2013. Exposed Science: Genes, the Environment, and the Politics of Population Health. University of California Press.
Vogel, Sarah A. 2013. Is It Safe? BPA and the Struggle to Define the Safety of Chemicals. University of California Press.
Sources
Ames, Bruce N. 1973. “Carcinogens are Mutagens: Their Detection and Classification.” Environmental Health Perspectives 6: 115–18.
Ames, Bruce N., Harold O. Kammen, and Edith Yamasaki. 1975. “Hair Dyes Are Mutagenic: Identification or a Variety of Mutagenic Ingredients.” Proceedings of the National Academy of Sciences, USA 72, no. 6: 2423–27.
Ames, Bruce N., Joyce McCann, and Edith Yamasaki. 1975. “Methods for Detecting Carcinogens and Mutagens with the Salmonella/Mammalian-Microsome Mutagenicity Test.” Mutation Research 31: 347–64.
Carson, Rachel. 1962. Silent Spring. Houghton Mifflin.
Environmental Defense Fund. 1997. Toxic Ignorance: The Continuing Absence of Basic Health Testing for Top-selling Chemicals in the United States. https://www.edf.org/sites/default/files/243_toxicignorance_0.pdf
McCann, Joyce, Edmund Choi, Edith Yamasaki, and Bruce N. Ames. 1975. “Detection of Carcinogens as Mutagens in the Salmonella/microsome Test: Assay of 300 Chemicals.” Proceedings of the National Academy of Sciences, USA 72, no. 12: 5135–39.
Websites and other sources
The (Un)making of Environmental Carcinogens: https://unmakingenvironmentalcarcinogens.princeton.edu
Distillations podcast on the Ames test: https://sciencehistory.org/stories/distillations-pod/the-ames-test/