—The explosion of air, the decomposition of water and the combustion of fire.—
In the years during which the outbreak of the French Revolution was brewing, explosions created in laboratories by experimenters in different European cities were about to break down the four elements on which the explanations of matter and its transformations had been based since classical antiquity. Air had been broken down into a growing number of elastic fluids – the ‘airs’ – with which famous experimenters from Italy, Britain or France and other less famous ones in many European countries were experimenting to find out their physical and chemical properties and determine their nature. These were new phenomena that for a time were explained by concepts that were already old. Finding out what their other colleagues were doing, observing and saying in their respective labs was just as important to these experimenters as making their own observations and interpretations reach others. Letters and publications circulated, but drawing and writing were poorly adapted when it came to describing these new phenomena, formalising still confusing explanations and, above all, replicating experiments made with strange instruments of changing designs and complicated to use. This is why travel was essential in these years of experiments and interpretations, continuing to be so when it was necessary to convince the most reluctant and the least interested colleagues of the validity of the explanations and demonstrations, which each one believed to have found in their cabinets and laboratories.
In those years, Alessandro Volta could be seen travelling halfway across Europe with his eudiometer; Charles Bladgen, Henry Cavendish’s collaborator, crossing the Channel to make his master’s pneumatic experiments known in Paris, comparing them with those of Antoine Lavoisier and Pierre-Simon Laplace; or travellers like the Portuguese Joao Jacinto de Magalhâes, touring several capitals to attend experimental demonstrations and to find out and then make known the novelties he had seen and heard elsewhere. Paris was an unmissable destination for all of them. The most skilled artisans who helped design and build the instruments were located there, and experimenters of Lavoisier’s stature ordered and paid for them. In Paris there was also the famous salon where Madame Lavoisier organised experimental demonstrations and discussions on the most surprising and new phenomena. This passage through Paris for all these experimental philosophers was crucial to what was about to happen in this city.
Apparatus used by Joseph Priestley in his pneumatic investigations in the 1770s. Wellcome Collection.
Many of the pneumatic chemists who were researching the composition of atmospheric air had found that by igniting a spark inside Volta’s eudiometer and causing the explosive combination of flammable air and dephlogisticated air (which a few years later would respectively be called hydrogen and oxygen), colourless, odourless and tastelessdrops that ‘looked like pure water’ formed on the walls of the vessel. Within the existing explanatory framework, many thought that water must be in the reacting airs, these being therefore mixtures of water and phlogiston in different proportions. Others devised ways to dehydrate the air and suggest the transmutation of air into water as a possible explanation. The old interpretations were still able to accommodate the new phenomena.

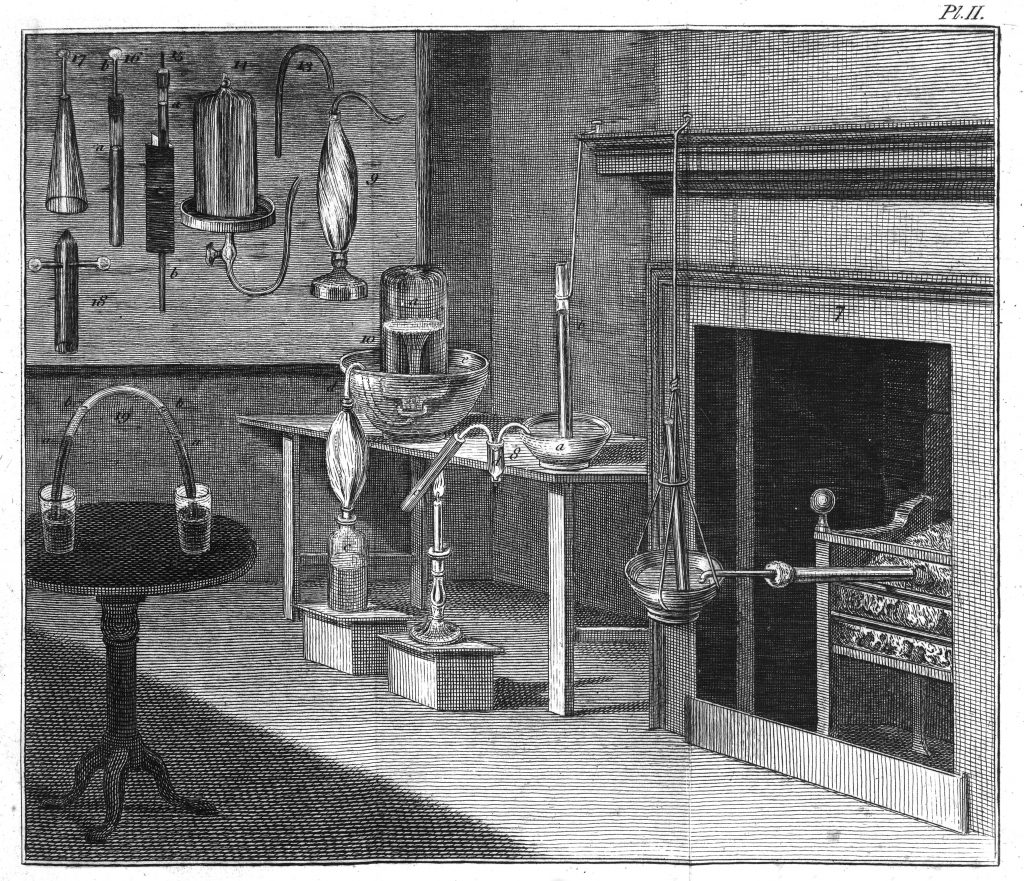
Recreation of Lavoisier’s laboratory at the Musee National des Arts et Métiers in Paris. Wikimedia.
Analysis and synthesis were the fundamental operations in chemistry, known and recognised as such by all who practised this science and, for some, art. Lavoisier supported his ideas on these to argue that, if it were possible to break down water into flammable air and vital air and then synthesise this back into water from those two elastic fluids, there would be no doubt that water was a compound and that these two airs were its two components. Something so easy to state proved much more complicated to do and, no less complicated to make convincing. Lavoisier mobilised all the material and human resources at his disposal to design a crucial experiment that would leave no doubt. The prestigious instrument maker Pierre Mégnié converted the designs made by the team of experimenters, made up of Lavosier, Monge, Laplace and the military engineer Jean-Baptiste Meusnier, into brass and glass apparatus. Into the experimental design, Lavoisier incorporated a technique learned in his work as a tax collector: balance sheets. Converted into mass balances, this accounting method was used to demonstrate that the quantities of product coincided with the sum of the reactants, without anything escaping or being added.
After several months having been spent on the construction and adjustment of the apparatus, verification of the results and public demonstrations in various private spaces, at the end of February 1785, Lavoisier gathered about thirty chemists, dignitaries and commissioners chosen by the Academy of Sciences in his laboratory at the Arsenal and he carried out, before their attentive gaze, a public demonstration of the experiments on the analysis and synthesis of water. Those summoned saw how the water decomposed in the apparatus designed by Meusnier and how the two gases released were carefully led to the new gasometers, a fundamental part of the device, designed by Meusnier and made by Mégnié, with which they were able to calculate the quantities of gases, measured by sophisticated precision scales. Through tubes controlled by valves they saw the gases being led to a spherical vessel in which two electrodes had been placed. The spark triggered combustion and the attendees saw how the spherical container was filled with that odourless, colourless and tasteless liquid that looked so much like water. After two whole days of experiments and calculations, Lavoisier demonstrated to the attendees that 100 parts of water had given 82 parts of “vital air” (our current oxygen) and 18 parts of “inflammable air” (our current hydrogen), while 86 parts of “vital air” had been combined with 14 parts of “inflammable air” to give 100 parts of what he claimed to be water.
The experiment, however spectacular and conclusive it seemed, was far from convincing everyone. In fact, it gave rise to a wide range of interpretations of the most disparate kind. For Lavoisier and his collaborators, the acidity of the water obtained by synthesis was due to possible impurities. For other chemists and commentators, the acidity showed that what was produced in this reaction was not water, but nitric acid. The differences observed between the proportions of the gases produced in the analysis and those consumed during the synthesis were also controversial. Others, while agreeing with the results, thought that they could be perfectly explained by current theories, without it being necessary to change them for others founded on pure conjecture. There were, finally, those who doubted the reliability of results that only Lavoisier was able to produce with sophisticated and expensive instruments, out of reach for the majority of the rest of experimenters.

Pneumatic chemistry interested different audiences in the 19th century, as illustrated by this caricature by James Gillray of Humphry Davy’s pneumatic experiments at the Royal Institution (1802). Wellcome Collection.
This resistance, as well as support, were reactions to very different circumstances and interests. National sentiments played an important role in the widespread opposition of German chemists, clinging to the theory of their revered Georg Stahl. The new theories also opened a generational gap, confronting those who saw the collapse of understanding with which they had worked for decades, and those who saw in those new ideas a way to grasp power from their elders. Nor did the different scientific and professional communities, many of which were dealing with matters far removed from those under discussion, respond in the same way. In the resolution of this dispute, instruments and strategies of persuasion were very important. Every ‘conversion’ of a prestigious chemist was announced as a victory for the ‘new chemistry’ against the old theories, in a battle for scientific and academic authority. Conversations and demonstrations with travellers passing through Paris or open public courses in the capital were key to reaching other territories with the new ideas, as was the writing of new textbooks, which ensured that new chemistry practitioners were instructed in the new explanatory framework from the outset. It was in that field of teaching and the spread of information that the simplified versions of the crucial instruments and experiments that have reached our current textbooks were designed.
Antonio García Belmar
IILP-UA
How to cite this paper:
García Belmar, Antonio. Travelling experiments. Sabers en acció, 2020-12-28. https://sabersenaccio.iec.cat/en/travelling-experiments/.
Find out more
You can find further information with the bibliography and available resources.
Recommended reading
Abbri, F. (1984), Le Terre, l’Acqua, le Arie. La revoluzione chimica del Settecento, Bologna, Il Mulino.
Bertomeu Sánchez, José R.; Antonio García Belmar. La Revolución Química: Entre la historia y la memoria. Valencia: PUV; 2006.
Donovan, A. (1988), The Chemical Revolution: Essays in Reinterpretation, Osiris, 4, 1-236.
Golinski, J. (2003), Chemistry. In: R. PORTER (ed.), Cambridge History of Science – Eighteenth-Century Science, Cambridge, Univ. Press, 375-397.
Holmes, F. (1990), Eighteenth-Century Chemistry as an Investigate Enterprise, Berkeley, University of California.
Klein, U. (1994), Origin of the concept of chemical compound, Science in context, 7, 163-204.
Mieli, A. (1948), Lavoisier y la formulación de la teoría química moderna, Buenos Aires-Mexico.
Studies
Bensaude-Vincent, B. (1983), A Founder Myth in the History of Sciences? The Lavoisier Case. In: L. Graham; W. Lepenies; P. Weingart, Functions and Uses of Disciplinary Histories, Dordrecht, D. Reidel Publishing Company, 53-79.
Bensaude-Vincent, B. (1993), Lavoisier. Mémoires d’une révolution, Paris, Flammarion.
Bertomeu Sánchez, José R.; García Belmar, Antonio, Visiones de la revolución química (1794-1943): entre la historia y la memoria, Cuadernos dieciochistas, 7, 113-140, 2006.
Carneiro, A.; Simoes, A: Diogo, M.P. (2000), Enlightenment Science in Portugal: The Estrangeirados and Their Communication Networks, Social Studies of Science, 30 (4), 591-619.
Crosland, M. (1983), A Practical Perspective on Joseph Priestley as a Pneumatic Chemist, British Journal for the History of Science, 16, 223-238.
Donovan, A. (1993), Antoine Lavoisier. Science, Administration and Revolution, Oxford, Blackwell Publishers.
Garcia Belmar, A.; Bertomeu Sanchez, J.R. (2001), Viajes a Francia para el estudio de la química, 1770-1833, Asclepius, 53 (1), 95-135.
Golinski, J. (1994), Precision Instruments and the Demonstrative Order of Proof in Lavoisier’s Chemistry, Osiris, 9, 30-48.
Holmes, F.; Levere, T. H. (eds.) (2000), Instruments and Experimentation in the History of Chemistry, Cambridge, MIT Press.
Klein, U. (1995), Fundamental Concepts of Early Modern Chemistry in the Context of the Operational and Experimental Practice, Berlin, MPIWG Preprints.
Levere, T. (2005), The role of instruments in the dissemination of the Chemical Revolution, Endoxa, 19, 227-242.
Perrin, C. (1973), Lavoisier, Monge and the Synthesis of Water. A Case of Pure Coincidence?, British Journal for the History of Science, 6 , 424-428.
Schaffer, S. (1990), Measuring Virtue: Eudiometry, Enlightenment and Pneumatic Medicine. In: A. CUNNINGHAM AND R. FRENCH (eds.), The Medical Enlightenment of the Eighteenth Century, Cambridge, University Press, 281-318.
Siegfried, R. (1988), The Chemical Revolution in the History of Chemistry, Osiris, 4, 34-53.
Sources
Lavoisier, Antoine-Laurent de, Traité élémentaire de chimie , présenté dans un ordre nouveau, et d’après les découvertes modernes ; Avec figures ; Par M. Lavoisier, A Paris, chez Cuchet, libraire, rue & hôtel Serpente. Sous le privilège de l’Académie des sciences & de la Société royale de médecine, 1789. Available here.
Pages of the class booklet in which the Pharmacy student Nicolas-Jean-Baptiste-Gaston Guibourt wrote down and drew the experimental demonstration of the decomposition and recomposition of water shown in the amphitheatre of the Collège de France by Jacques Louis Thenard in the early nineteenth century (BibliothèqueInteruniversitaire de Pharmacie de Paris, Mss. 22 – 23, Vol. 1, 118-119. Available here. Page 118 available here. Page 199 available here.



